
|
Synonym(s)
Classification
(Guiry and Guiry 2011)
Lifestyle
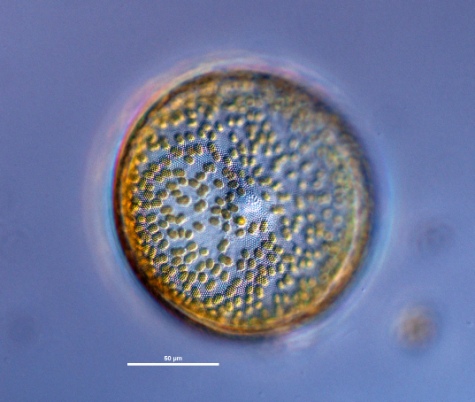
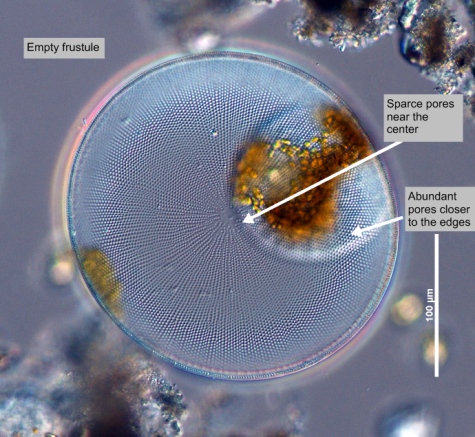
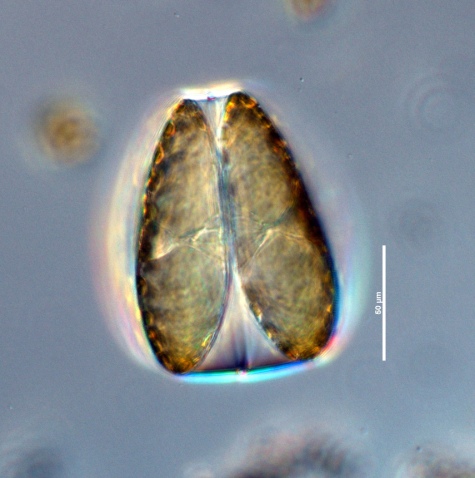
Description
Girdle
In diatoms, the portion of the cell wall between the two valves of a cell; made up of intercalary bands (bands closest to the valves) and connecting bands (bands in the middle of the girdle). In dinoflagellates, the equivalent of a cingulum or transverse furrow (Horner 2002).
girdle view with one side higher than the other (may not be visible in all orientations). CloseValve
In diatoms, the structurally distinct halves of the cell wall (Becker 1996).
Valves are most convex at the tallest part of the cell, near one end of the cell. Cells have a rosette of larger CloseAreola(e)
In diatoms, the regularly repeated hexagonal holes on the valve walls (HPP 2003).
areolae at the centre of the valve. Chloroplasts are smooth and disc-shaped (Hasle and Syvertsen 1997). Cells are yellow-brown in colour.Margin
The outline or border that defines the shape of an organism or cell.
margin, and 11 near margin; on edge of valve CloseMantle
In diatoms, "the part of a valve that extends from the valve face, forming the valve edge." It is visible when the frustule is viewed in girdle view (Spaulding 2010).
mantle 13 in 10 μm. Chamber openings small, dotlike. Outer closing membrane of areolae very delicately ClosePoroid
A simple hole through the surface of a diatom valve (Smithsonian 2011).
poroid. CloseRadial
(symmetry) Describing a shape that many axes of symmetry. That is, it does not have a left and right like humans do (bilateral symmetry), but can be divided into equal halves no matter where you place the axis. Some examples of radially symmetrical organisms include sea stars and centric diatoms like Thalassiosira.
Radial rows and secondary spiral rows distinct. Marginal CloseSpinula
(plural: spinulae) A small spine or hook.
spinulae and the CloseHyaline
Resembling glass; transparent or translucent.
hyaline lines radiating from the spinulae toward the center distinct, 5 - 7 μm apart. Two small CloseProcess
A natural projection or appendage on an organism.
processes or CloseApiculus
(plural: apiculi) Short, sharp, but not stiff projections (Kuo 2006).
apiculi on margin at distance of about 120° from each other. Girdle formed from the two similar CloseGirdle bands
The connecting elements between two valves of a diatom cell. "They enclose and protect the cell and also accommodate the increase in cell volume during the cell cycle" (Horner 2002).
girdle bands. No CloseIntercalary bands
Girdle bands that are furthest away from the valve (Smithsonian 2011).
intercalary bands" (Cupp 1943).Valvocopula
In diatoms, a band next to the valve that may help link the valves together (Hasle and Syvertsen 1996).
"Valvocopula wedge shaped, widest opposite to the opening. Radial CloseAreolation
Often used to describe holes (areolae) on the valve surface of diatom frustules.
areolation, incomplete CloseStriae
(referring to pores in diatoms) In diatoms, a striation or row of pores on the valve face. "In centric diatoms, striae may be radial, running from the centre of the valve to the margin ... In pennate diatoms, striae may be parallel to the median line of the valve or raphe" (Horner 2002).
striae, and CloseDecussating
Intersecting to form an"X" shape.
decussating arcs in the central part of the valve. CloseCribrum (cribra)
(plural: cribrae) A perforated siliceous plate that blocks one of the many pores in a diatom's frustule (van den Hoek et al. 1995).
Cribra barely discernible with CloseLM
(light microscopy) "Using a microscope in which a beam of light passes through optical lenses to view an image of the specimen" (MCM LTER 2010).
LM. One ring of CloseMarginal process
In some diatoms, a long, coarse external tube through the frustule (Tomas 1997).
marginal processes including two larger processes around 135° apart, readily seen with LM; the larger processes seen as deep indentations of the valve mantle. Hyaline lines from the marginal processes toward the valve centre more or less distinct" (Hasle and Syvertsen 1997).Measurements
Diameter: 40 - 200 μm
Height Close
Pervalvar axis
The axis through the centre point of the two valves of a frustule. This axis is perpendicular to the valve face.
(pervalvar axis): 30 - 180 μm at widest part (Kraberg et al. 2010)Valve areolae: 8 - 11 in 10 μm
No. of bands/ Close
Theca
(plural: thecae) Cell wall. In dinoflagellates, it is composed of cellulose plates within vesicles (Horner 2002).
theca: 2Valvocopula width: 17 - 20 μ
(Hasle and Syvertsen 1997)
Margin rings: 1
Areolae from margin: 3 - 4
Areolae apart: 2 - 5
(Hasle and Syvertsen 1997)
Similar species
Harmful effects
Habitat
Distribution
Cosmopolitan (Kraberg et al. 2010).
Forms characteristic autumn blooms in the Baltic Sea (Wasmund et al. 2003). Present throughout the year in "low to moderate" abundance, with higher concentrations in spring and summer in North European Seas (Kraberg et al. 2010).
"Not uncommon off California. Greatest abundance in Mission Bay near San Diego. North temperate or Close
Boreal
Relating to the area immediately south of the Arctic.
boreal species." (Cupp 1943).Growth conditions
Silicic acid
A general term to describe chemical compounds containing silicon, oxygen and hydrogen with a general formula of [SiOx(OH)4-2x]n. Diatoms polymerize silicic acid into biogenic silica to form their frustules (Azam and Chisholm 1976).
Silica uptake may be inversely correlated with light intensity to maintain a stable growth rate (Taylor 1985). May be more prone to parasite infections during summer to autumn months, when water temperatures range around 13 - 20 °C (Wetsteyn and Peperzak 1991). Also, potential blooms may be suppressed during turbulent conditions, where infections persistently keep concentrations low (Kühn and Hofmann 1999).Irradiance
Amount of solar energy per unit area on a surface (units: μE m-2 sec-1, where E is an Einstein, a mole of photons).
irradiance (Kühn 1998), or during conditions of high pH (above ∼8.8; Kühn and Köhler-Rink 2008).Environmental Ranges
Temperature range (°C): 14.227 - 29.468
Nitrate (μmol L-1): 0.056 - 2.197
Salinity (PSU): 33.919 - 36.252
Oxygen (mL L-1): 4.500 - 5.952
Phosphate (μmol L-1): 0.062 - 0.279
Silicate (μmol L-1): 1.145 - 7.894
(OBIS 2011, cited in EOL 2011)
Bloom characteristics
References
Baudin, J. P. 1980. A contribution to the ecological study of Mediterranean brackish water systems. 2. Populations of the Citis lagoon (Bouches du Rhone). Vie et milieu. Paris. 30(3-4): 303-308.
Cupp, E. E. 1943. Marine Plankton Diatoms of the West Coast of North America. University of California Press. Berkeley, California. 238.
Encyclopedia of Life (EOL). 2011. Coscinodiscus granii. http://eol.org/pages/912736/overview. Accessed 8 Oct 2011.
Guiry, M. D. and Guiry, G. M. 2011. Coscinodiscus granii Gough. http://www.algaebase.org/search/species/detail/?species_id=37690. Accessed 8 Oct 2011.
Hasle, G. R. and Syvertsen, E. E. 1997. Marine diatoms. In: Tomas, C. R. (ed.) Identifying marine Phytoplankton. Academic Press, Inc., San Diego. 5-385.
Horner, R. A. 2002. A Taxonomic Guide To Some Common Phytoplankton. Biopress Limited, Dorset Press, Dorchester, UK. 200.
Kraberg, A., Baumann, M. and Durselen, C. D. 2010. Coastal Phytoplankton: Photo Guide for Northern European Seas. Verlag Dr. Friedrich Pfeil, Munchen, Germany. 204.
Kühn, S. F. 1998. Infection of Coscinodiscus spp. by the parasitoid nanoflagellate Pirsonia diadema: II. Selective infection behaviour for host species and individual host cells. Journal of Plankton Research. 20(3): 443-454.
Kühn, S. F. and Hofmann, M. 1999. Infection of Coscinodiscus granii by the parasitoid nanoflagellate Pirsonia diadema: III. Effects of turbulence on the incidence of infection. Journal of Plankton Research. 21(12): 2323-2340.
Kühn, S. F. and Köhler-Rink, S. 2008. pH effect on the susceptibility to parasitoid infection in the marine diatom Coscinodiscus spp. (Bacillariophyceae). Marine Biology. 154(1): 109-116.
Ocean Biogeographic Information System (OBIS). 2011. Coscinodiscus granii. http://www.iobis.org/mapper/?taxon_id=427704. Accessed 8 Oct 2011.
Rabsch, U. and Elbrächter, M. 1980. Cadmium and zinc uptake, growth, and primary production in Coscinodiscus granii cultures containing low levels of cells and dissolved organic carbon. Helgoland Marine Research. 33(1-4): 79-88.
Schmid, A. M. M. 1995. Sexual reproduction in Coscinodiscus granii Gough in culture: a preliminary report. Proceedings of the thirteenth International Diatom Symposium, Maratea, Italy, 1st-7th September 1994. 139-159.
Taylor, N. J. 1985. Silica incorporation in the diatom Coscinodiscus granii as affected by light intensity. British Phycological Journal. 20(4): 365-374.
Wasmund, N., Pollehne, F., Postel, L., Siegel, H. and Zettler, M. L. 2003. Biological state assessment of the Baltic Sea in 2002. Institut fuer Ostseeforschung, Warnemuende (Germany). 78.
Wetsteyn, L. P. M. J. and Peperzak, L. 1991. Field observations in the oosterschelde (The Netherlands) on Coscinodiscus concinnus and Coscinodiscus granii (Bacillariophyceae) infected by the marine fungus Lagenisma coscinodisci (Oomycetes). Hydrobiological Bulletin. 25(1): 15-21.
